Transcraneal Magnetic Stimulation
Discover the Difference
TMS Therapy for Depression

A global mental
health crisis
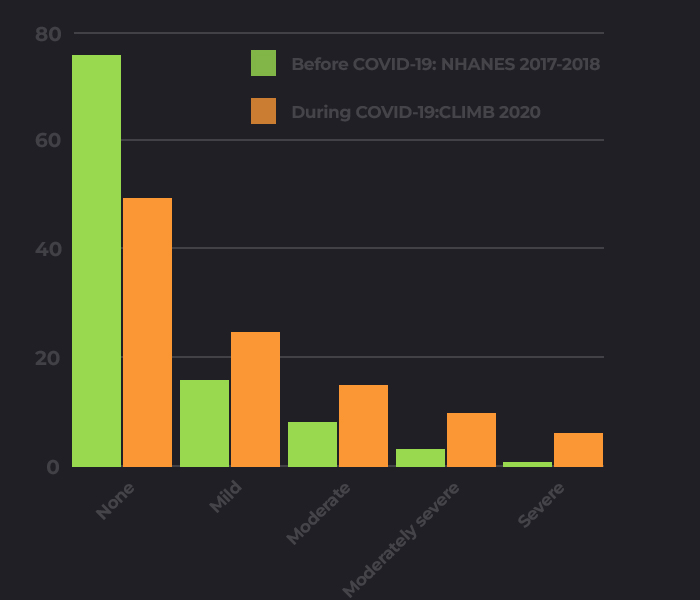
Before COVID there were over 1 billion people
suffering with Depression
worsened by
the pandemic
worsened
by the
pandemic
The mental fallout will stay long beyond COVID
Large Patient Population
Being Unserved
16.1 Million US Adults with Major Depression
7.2 Million Treated
4.5 Million Poorly Served
548,000
Americans, per day, call in
sick for mental health
issues, costing 200 million
work days per year
Major Depression in the Brain
PET Scans showing the activity of a brain of patient with depression vs. a patient with no depression
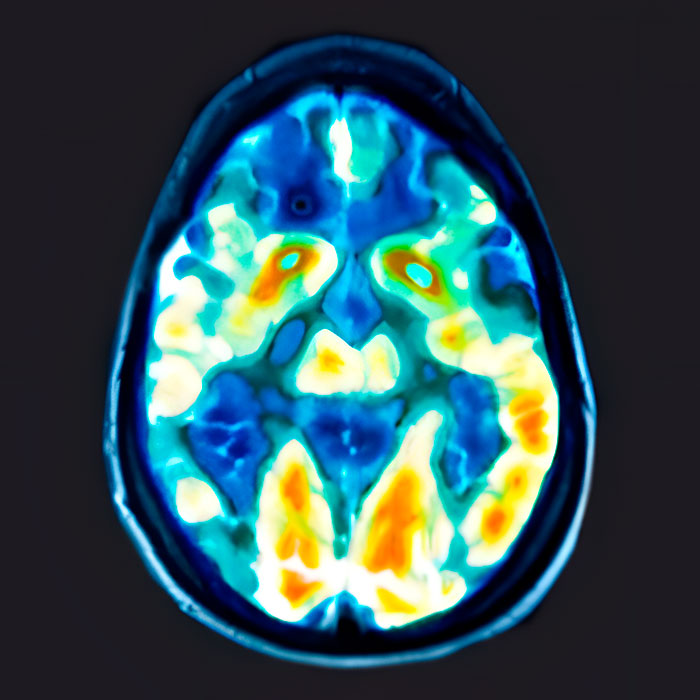
Depression
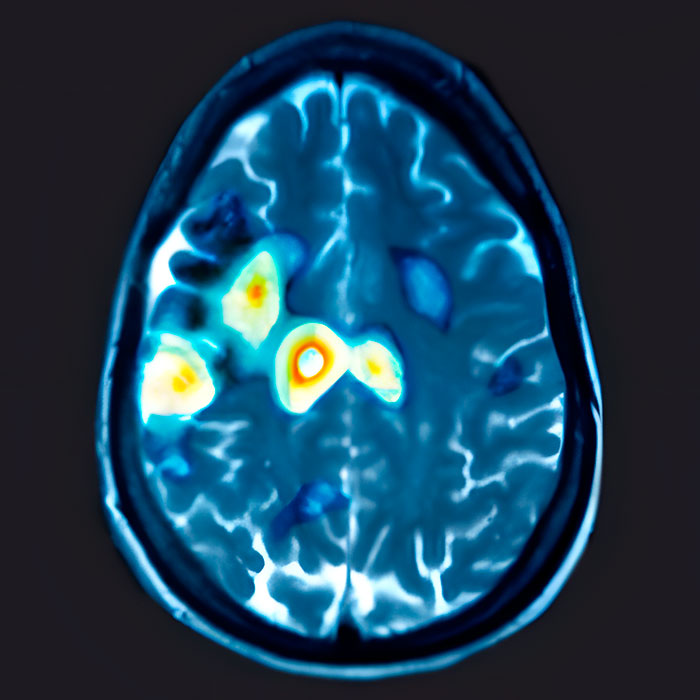
No Depression
TMS Increases Brain Activity
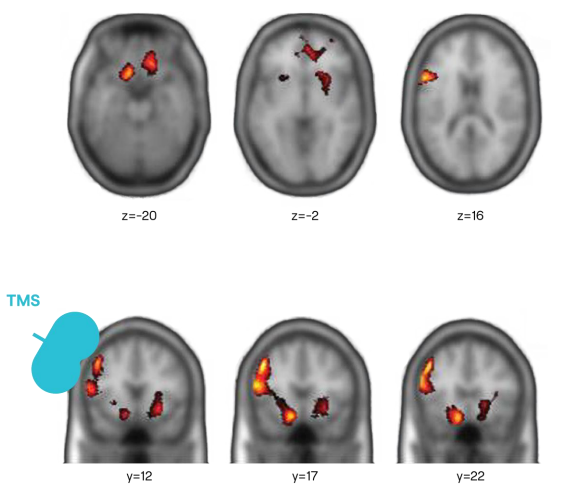
Functional MRI showing new brain activity post TMS in 6 cross section scans
The Brain is an Electrochemical Organ
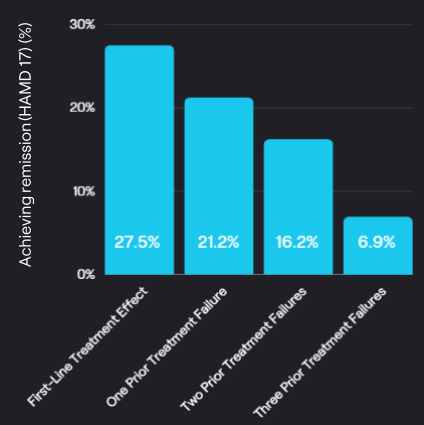
STAR*D Study
demonstrates that current treatment has limited effectiveness
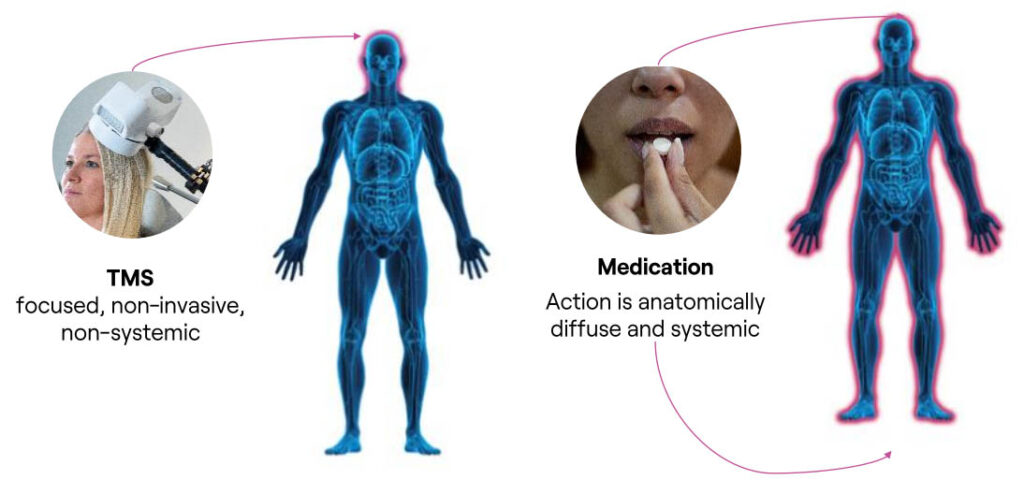
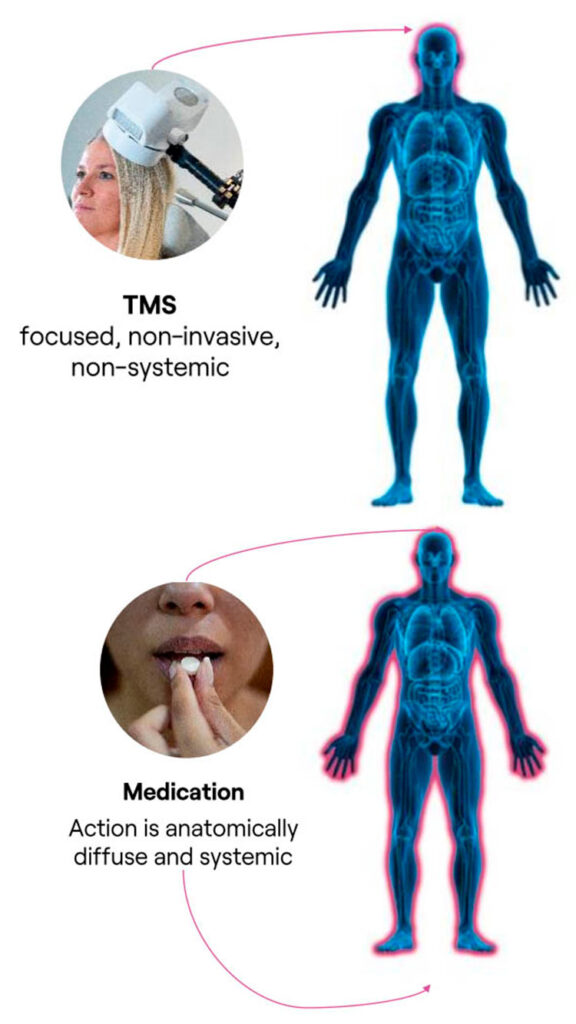
TMS Therapy Avoids the Systemic Side Effects Commonly Experienced with Medications
Systemic Drug Side Effects
- Weight Gain
- Constipation
- Diarrhea
- Nausea
- Drowsiness
- Insomnia
- Nervousness
- Increased Appetite
- Decreased Appetite
- Decreased Sexual Interest
- Anxiety
- Weakness
- Dry Mouth
- Dizziness
- Fatigue
- Headache/ Migraine
- Abnormal Ejaculation
- Impotence
- Sweating
- Tremor
- Treatment Discontinuation Side Effects
What is TMS?
TMS stands for Transcranial Magnetic Stimulation and is a well-tolerated and proven method to treat Depression, even in patients who have not responded to other forms of therapy.
- TMS works by positioning a magnetic coil on your head which externally stimulates specific areas of the brain associated with depression symptoms.
- The coil emits a light stimulation aimed at specific areas within the brain which feels like a tapping sensation.
- The magnetic stimulation encourages the brain cells to naturally release the chemicals necessary to properly regulate our mood.

The TMS Magnetic Field is similar to an MRI
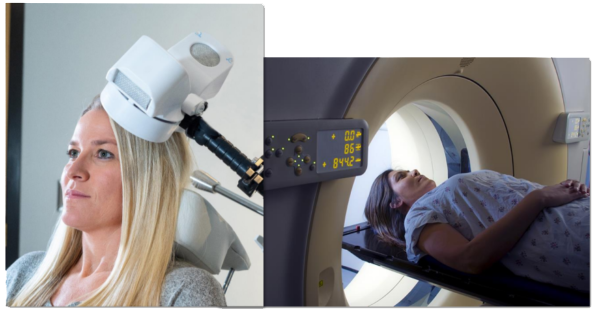
TMS Therapy System
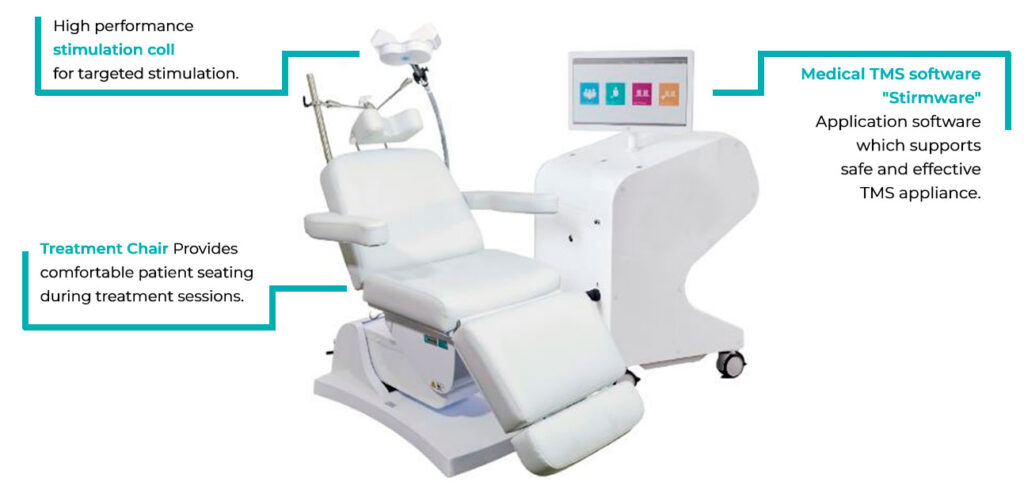

Advantages of TMS Therapy
Natural
TMS therapy uses electro-magnetic pulses to stimulate brain cells which helps to modulate neural pathways necessary for mood regulation.
A medication-free approach
TMS is particularly suited for people who do not respond to antidepressant medications for their treatment of depression.
No hospitalization or anesthetic required
TMS is locally applied and a gentle method of stimulation. For this reason there is no need for sedation or anesthesia (unlike, for example, ECT (Electroconvulsive Therapy))
Minimal side-effects
Unlike some antidepressant medications, TMS treatment does not cause weight gain, sexual dysfunction, nausea, negative cognitive effects or withdrawal symptoms. Patients may experience a focal muscle tension headache immediately after a sessions, but this is usually relieved with a mild painkiller.
A short-term program
Patients normally notice improvements within the first few sessions. For most clints a long-term positive effective is reached after 20-40 sessions, which can be completed in a few weeks or months.
FDA Approved
TMS is a safe form of treatment that has been cleared by the FDA since 2008 for patients with depression who have failed to achieve satisfactory Improvement from prior antidepressant medication.
A typical course of TMS
- Up to 36 x 19-minute sessions over several weeks.
- Although treatment requires regular visits, this is just in a short space of time.
- Long lasting effects are usually noticed after the course of treatment.
- Patients can resume normal activities following their treatments.
Covered by most insurance plans

Who Can Benefit from TMS Therapy?
- patients 18 years and over, diagnosed with depression.
- patients who have not responded to other treatments and have been diagnosed with treatment-resistant depression.
- patients who wish to lower or cease medication.
- for depression and want a therapy to support that process.
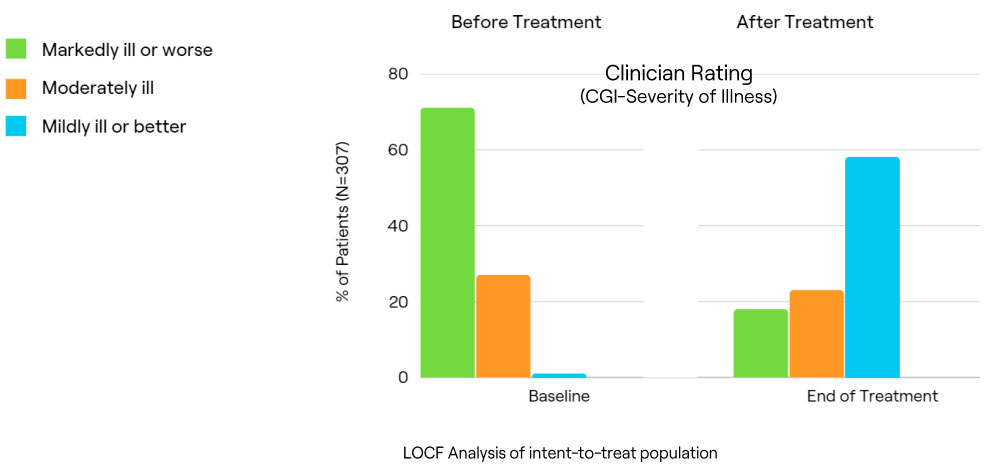
Comparison of End of Acute Treatment Clinical Status
Clinician Assessed Outcomes
TMS in Clinical Practice
FDA Cleared
Non-drug
Non-invasive
No side effects of drugs
Long-lasting symptom relief
Covered by most insurance
Who Can Benefit from TMS Therapy?
- Has your Doctor recommended TMS treatment for you?
- O Have you been advised of the insurance coverage you may have for TMS treatment, including what it does / does not cover?
- Have payment options been outlined to you?
- Do you have your initial consultation already booked?
Menu
Contact
- 1641 W Flagler St Miami, FL 33135
- +1 (786) 360-3213
- +1 (407) 350-4529
- admin@royalhealthcenters.com


